Makrolife´s Consulting Services
Services – End-to-End Healthcare Consulting & Execution
Macrolife Biotech offers
End-to-end consulting and operational implementation for companies in the areas
Healthcare, Life Sciences, Biotechnology, Cosmetics and Dietary Supplements.
We accompany products
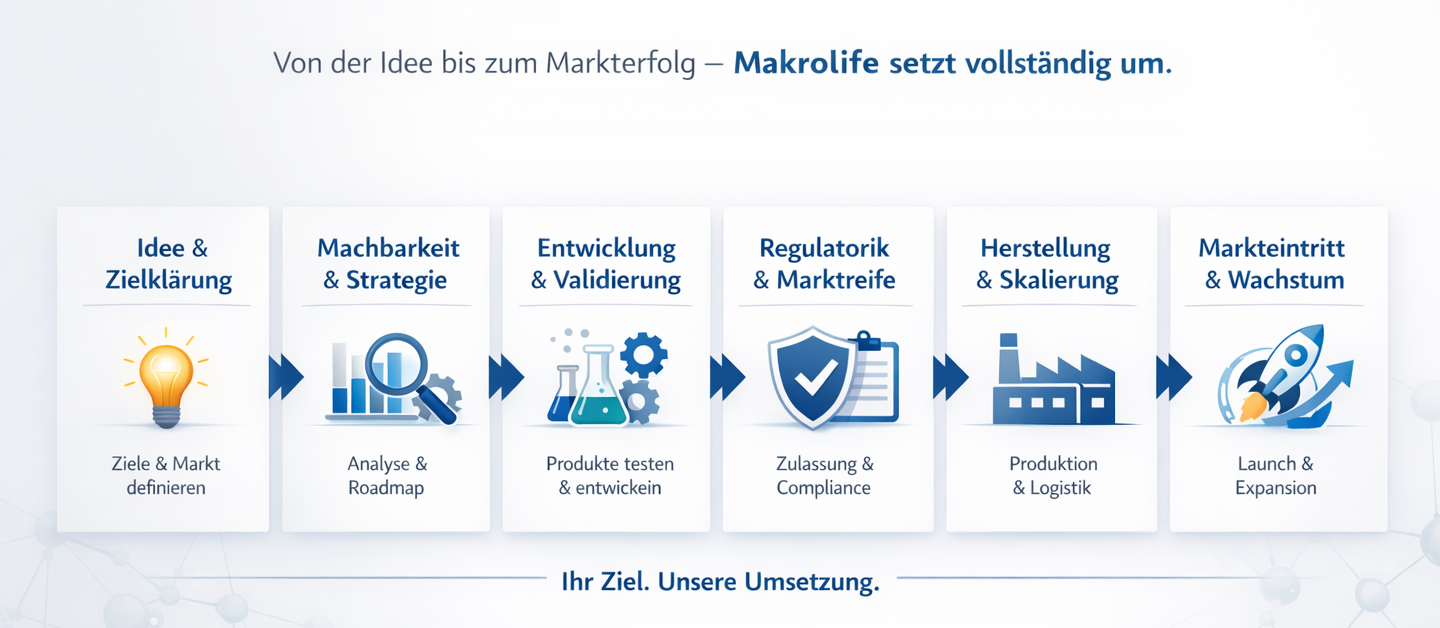
from idea to scalable market success – structured, regulatory compliant, and internationally oriented. Unlike traditional consulting, our work doesn't end with concepts or presentations. Makrolife Biotech takes on the
Full implementation throughout the product lifecycleStrategy, product development, validation, regulatory compliance, manufacturing and market entry.
Our customers define the goal – we deliver.
Planning, implementation and control.
Strategy & Feasibility Analysis
Product Development & Recipe Design
Validation & Testing
Regulatorik & Compliance
Manufacturing & Scaling
Market Entry & Growth
Our services are aimed at Entrepreneurs, brand owners, investors and decision-makers, which about Capital, clear market opportunities, or solid product ideas possess, however, a Reliable end-to-end implementation in the healthcare and life sciences environment Macrolife Biotech fills precisely this gap and assumes full responsibility throughout the entire product lifecycle.
In contrast to isolated consulting, Makrolife Biotech stands for End-to-end responsibility with operational implementationOur work is based on deep regulatory and scientific expertise, combined with clearly structured processes and international implementation expertise. We manage projects securely through development, validation, regulatory compliance, manufacturing, and market entry – transparent, measurable and scalableMacrolife Biotech stands for structured, results-oriented implementation in the healthcare sector and consistently accompanies products from idea to market.
End-to-end means responsibility
Makrolife Biotech doesn't just provide guidance – we deliver results
“Many of our clients have capital, a strong idea, or a clear market opportunity – but no time to struggle through complex development, testing, and regulatory processes. That’s exactly where we come in.”
At Makrolife Biotech, we don't just provide consulting services, we take full responsibility for implementation. We structure projects, make well-informed decisions, manage development, validation, regulatory compliance, and manufacturing, and guide products safely to market readiness.
Our goal is to reduce complexity, control risks, and turn an idea into a marketable, scalable product.
Dr. Martin Großherr
Senior Consultant, Makrolife Biotech







